Question:
For a reaction scheme $\mathrm{A} \stackrel{\mathrm{k}_{1}}{\longrightarrow} \mathrm{B} \stackrel{\mathrm{k}_{2}}{\longrightarrow} \mathrm{C}$, if
the rate of formation of B is set to be zero then the concentration of $B$ is given by :
Correct Option: 1
Solution:
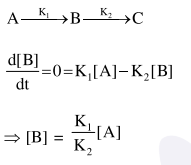
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
