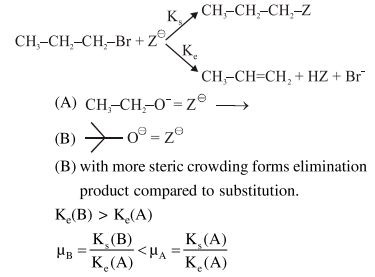
Question: For the following reactions:
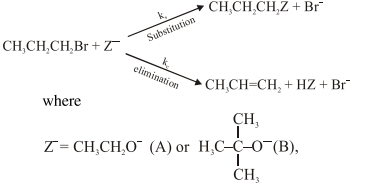
$\mathrm{k}_{\mathrm{s}}$ and $\mathrm{k}_{\mathrm{e}}$, are , respectively, the rate constants for
the substitution and elimination, and $\mu=\frac{\mathrm{k}_{\mathrm{s}}}{\mathrm{k}_{\mathrm{e}}}$, the correct options is -
$\mu_{\mathrm{B}}>\mu_{\mathrm{A}}$ and $\mathrm{k}_{\mathrm{e}}(\mathrm{B})>\mathrm{k}_{\mathrm{e}}(\mathrm{A})$
$\mu_{\mathrm{B}}>\mu_{\mathrm{A}}$ and $\mathrm{k}_{\mathrm{e}}(\mathrm{A})>\mathrm{k}_{\mathrm{e}}(\mathrm{B})$
$\mu_{\mathrm{A}}>\mu_{\mathrm{B}}$ and $\mathrm{k}_{\mathrm{e}}(\mathrm{B})>\mathrm{k}_{\mathrm{e}}(\mathrm{A})$
$\mu_{\mathrm{A}}>\mu_{\mathrm{B}}$ and $\mathrm{k}_{\mathrm{e}}(\mathrm{A})>\mathrm{k}_{\mathrm{e}}(\mathrm{B})$
Correct Option: , 3
Solution: