Question:
For the reaction
$\mathrm{A}+\mathrm{B} \rightleftharpoons 2 \mathrm{C}$
the value of equilibrium constant is 100 at $298 \mathrm{~K}$.
If the initial concentration of all the three species is
$1 \mathrm{M}$ each, then the equilibrium concentration of $\mathrm{C}$ is $x \times 10^{-1}$ M. The value of $x$ is______
(Nearest integer)
Solution:
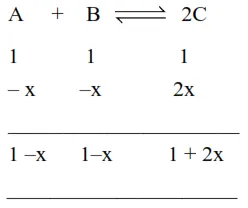
$\mathrm{K}=\frac{[\mathrm{C}]_{\mathrm{eq}}^{2}}{[\mathrm{~A}]_{\mathrm{eq}}[\mathrm{B}]_{\mathrm{eq}}}=\frac{(1+2 \mathrm{x})^{2}}{(1-\mathrm{x})(1-\mathrm{x})}$
$100=\left(\frac{1+2 x}{1-x}\right)^{2}$
$\left(\frac{1+2 x}{1-x}\right)=10$
$x=\frac{3}{4}$
$[\mathrm{C}] \mathrm{e}_{\mathrm{q} .}=1+2 \mathrm{x}$
$=1+2\left(\frac{3}{4}\right)$
$=2.5 \mathrm{M}$
$25 \times 10^{-1} \mathrm{M}$
