Question.
How will you find the valency of chlorine, sulphur and magnesium?
How will you find the valency of chlorine, sulphur and magnesium?
Solution:
(i) Atomic number of chlorine $=17$
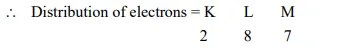
Thus, outermost shell has 7 electrons. It can easily complete its octet by gaining one electron. Hence, its valency = 1.
(ii) Atomic number of sulphur = 16
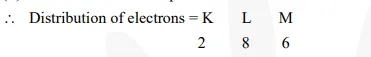
Thus, outermost shell has 6 electrons.
Hence, valency = 8 – 6 = 2
(iii) Atomic number of magnesium = 12\
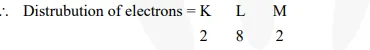
Thus, outermost shell has 2 electrons. It can easily complete its octet by loosing two electrons. Hence, its valency = 2.
(i) Atomic number of chlorine $=17$
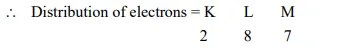
Thus, outermost shell has 7 electrons. It can easily complete its octet by gaining one electron. Hence, its valency = 1.
(ii) Atomic number of sulphur = 16
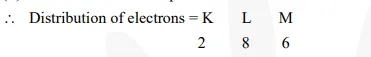
Thus, outermost shell has 6 electrons.
Hence, valency = 8 – 6 = 2
(iii) Atomic number of magnesium = 12\
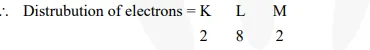
Thus, outermost shell has 2 electrons. It can easily complete its octet by loosing two electrons. Hence, its valency = 2.
