Question:
Identify the elements X and Y using the ionisation energy values given below :
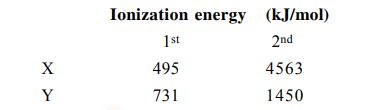
Correct Option: 1
Solution:
$\mathrm{Na} \rightarrow[\mathrm{Ne}] 3 \mathrm{~s}^{1} \mathrm{IE}_{1}$ is very low but $\mathrm{IE}_{2}$ is very high due to stable noble gas configuration of
$\mathrm{Na}^{+}$
$\mathrm{Mg} \rightarrow[\mathrm{Ne}] 3 \mathrm{~s}^{2} \mathrm{IE}_{1} \& \mathrm{IE}_{2} \rightarrow$ Low
$\mathrm{IE}_{3}$ is very high.
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
