Question.
In a reaction, 5.3 g of sodium carbonate reacted with 6 g of ethanoic acid. The products were 2.2 g of carbon dioxide, 0.9 g water and 8.2 g of sodium ethanoate. Show that these observations are in agreement with the law of conservation of mass.
In a reaction, 5.3 g of sodium carbonate reacted with 6 g of ethanoic acid. The products were 2.2 g of carbon dioxide, 0.9 g water and 8.2 g of sodium ethanoate. Show that these observations are in agreement with the law of conservation of mass.
Solution:
Sodium carbonate reacts with ethanoic acid converted into sodium ethanoate, carbon dioxide,and water
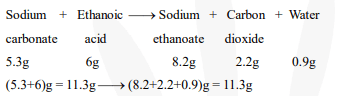
$\therefore$ Total mass before the reaction $=$ Total mass after the reaction
Hence, the given observations are in agreement with the law of conservation of mass.
Sodium carbonate reacts with ethanoic acid converted into sodium ethanoate, carbon dioxide,and water

$\therefore$ Total mass before the reaction $=$ Total mass after the reaction
Hence, the given observations are in agreement with the law of conservation of mass.
