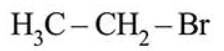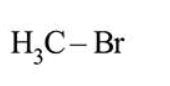Question:
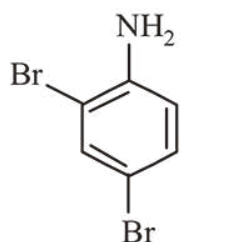
In Carius method of estimation of halogen, $0.172 \mathrm{~g}$ of an organic compound showed presence of $0.08 \mathrm{~g}$ of bromine. Which of these is the correct structure of the compound?
Correct Option: , 2
Solution:
Mole of bromine $=\frac{0.08}{80}=10^{-3} \mathrm{~mole}$
Molar mass of compound is given by the following equation,
$\frac{0.172}{M}=10^{-3}$
$\Rightarrow M=\frac{0.172}{10^{-2}}=172 \mathrm{~g}$
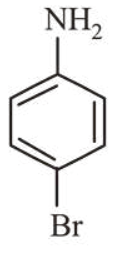
$\because$ Molar mass of $\mathrm{C}_{6} \mathrm{H}_{6} \mathrm{NBr}$
$=(6 \times 12)+(1 \times 6)+(1 \times 14)+(1 \times 80)=172 \mathrm{~g}$
Thus option (b) is the correct structure of compound.
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.