In the alkane $\mathrm{H}_{3} \mathrm{C}-\mathrm{CH}_{2}-\mathrm{C}\left(\mathrm{CH}_{3}\right)_{2}-\mathrm{CH}_{2}-\mathrm{CH}\left(\mathrm{CH}_{3}\right)_{2}$, identify $1^{\circ}, 2^{\circ}, 3^{\circ}$ carbon atoms and give the number of $\mathrm{H}$ atoms bonded to each one of these.
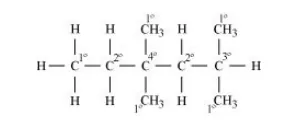
1° carbon atoms are those which are bonded to only one carbon atom, i.e., they have only one carbon atom as their neighbour. The given structure has five 1° carbon atoms and fifteen hydrogen atoms are attached to it.
2° carbon atoms are those which are bonded to two carbon atoms, i.e., they have two carbon atoms as their neighbours. The given structure has two 2° carbon atoms and four hydrogen atoms are attached to it.
3° carbon atoms are those which are bonded to three carbon atoms, i.e., they have three carbon atoms as their neighbours. The given structure has one 3° carbon atom and only one hydrogen atom is attached to it.
