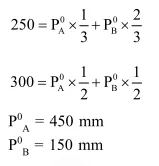Question:
Liquids A and B form an ideal solution. At $30^{\circ} \mathrm{C}$, the total vapour pressure of a solution containing $1 \mathrm{~mol}$ of $\mathrm{A}$ and 2 moles of B is $250 \mathrm{~mm} \mathrm{Hg}$. The total vapour pressure becomes $300 \mathrm{~mm} \mathrm{Hg}$ when 1 more mol of $\mathrm{A}$ is added to the first solution. The vapour pressures of pure $\mathrm{A}$ and $\mathrm{B}$ at the same temperature are
[JEE (MAIN)-2012 ONLINE]
Correct Option: 1
Solution: