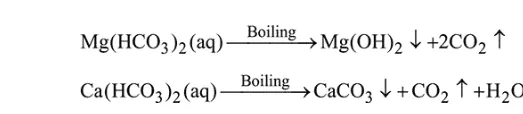
Question: The temporary hardness of a water sample is due to compound $X$. Boiling this sample converts $X$ to compound Y. X and Y, respectively, are :
$\mathrm{Mg}\left(\mathrm{HCO}_{3}\right)_{2}$ and $\mathrm{Mg}(\mathrm{OH})_{2}$
$\mathrm{Ca}\left(\mathrm{HCO}_{3}\right)_{2}$ and $\mathrm{Ca}(\mathrm{OH})_{2}$
$\mathrm{Mg}\left(\mathrm{HCO}_{3}\right)_{2}$ and $\mathrm{MgCO}_{3}$
$\mathrm{Ca}\left(\mathrm{HCO}_{3}\right)_{2}$ and $\mathrm{CaO}$
Correct Option: 1
Solution:
Temporary hardness is caused by bicarbonates of calcium and magnesium. On boiling following changes occurs,