n mole of a perfect gas undergoes a cyclic process ABCA (see figure) consisting of the following processes -
$\mathrm{n}$ mole of a perfect gas undergoes a cyclic process $\mathrm{ABCA}$ (see figure) consisting of the following processes -
$\mathrm{A} \rightarrow \mathrm{B}$ : Isothermal expansion at temperature $\mathrm{T}$ so that the volume is doubled from $\mathrm{V}_{1}$ to $\mathrm{V}_{2}=2 \mathrm{~V}_{1}$ and pressure changes from $\mathrm{P}_{1}$ to $\mathrm{P}_{2}$. $\mathrm{B} \rightarrow \mathrm{C}$ : Isobaric compression at pressure $\mathrm{P}_{2}$ to initial volume $\mathrm{V}_{1}$. $\mathrm{C} \rightarrow \mathrm{A}$ : Isochoric change leading to change of pressure from $\mathrm{P}_{2}$ to $\mathrm{P}_{1}$. Total workdone in the complete cycle $\mathrm{ABCA}$ is -
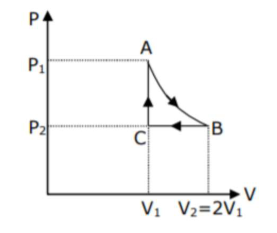
Correct Option: 4,
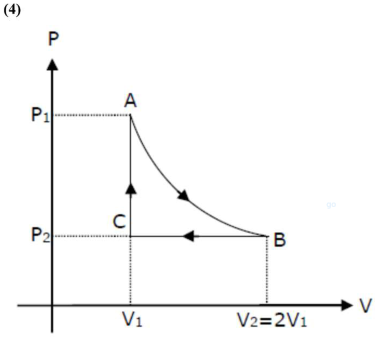
$A \rightarrow B=$ isotheraml process
$\mathrm{B} \rightarrow \mathrm{C}=$ isobaric process
$C \rightarrow A=$ isochoric process
also, $V_{2}=2 V_{1}$
work done by gas in the complete cycle $\mathrm{ABCA}$ is
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
