Question:
$\mathrm{NaClO}_{3}$ is used, even in spacecrafts, to produce $\mathrm{O}_{2}$. The daily consumption of pure $\mathrm{O}_{2}$ by a person is $492 \mathrm{~L}$ at 1 atm, $300 \mathrm{~K}$. How much amount of $\mathrm{NaClO}_{3}$, in grams, is required to produce $\mathrm{O}_{2}$ for the daily consumption of a person at 1 atm, $300 \mathrm{~K}$ ?
$\mathrm{NaClO}_{3}(\mathrm{~s})+\mathrm{Fe}(\mathrm{s}) \rightarrow \mathrm{O}_{2}(\mathrm{~g})+\mathrm{NaCl}(\mathrm{s})+\mathrm{FeO}(\mathrm{s})$
$\mathrm{R}=0.082 \mathrm{~L}$ atm $\mathrm{mol}^{-1} \mathrm{~K}^{-1}$
Solution:
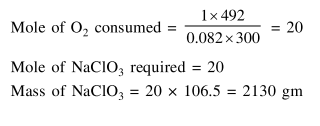
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
