On adding a drop of barium chloride solution to an aqueous solution of sodium sulphite, white precipitate is obtained :
(a) Write a balanced chemical equation for the reaction involved.
(b) What other name can be given to this precipitation reaction ?
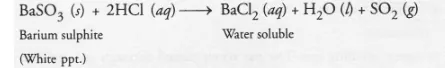
(c) On adding dilute hydrochloric acid to the reaction mixture, white precipitate disappears. Why ?
$\begin{aligned} \text { (a) } \mathrm{BaCl}_{2}(a q)+\mathrm{Na}_{2} \mathrm{SO}_{3}(a q) \longrightarrow & \mathrm{BaSO}_{3}(s)+2 \mathrm{NaCl}(a q) \\ & \text { (White ppt.) } \end{aligned}$
(b) The precipitation reaction is also called double displacement reaction.
(c) White precipitate of barium sulphite reacts with dilute hydrochloric to form barium chloride and sulphur dioxide gas. Since barium chloride is
water soluble, the white precipitate will slowly disappear.