Question:
On heating, lead(II) nitrate gives a brown gas (A). The gas (A) on cooling changes to a colourless solid/liquid (B). (B) on heating with $\mathrm{NO}$
changes to a blue solid $(\mathrm{C})$. The oxidation number of nitrogen in solid (C) is :
Correct Option: , 4
Solution:
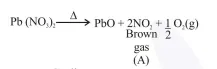
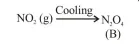
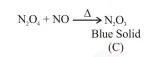
O.S. of nitrogen in $\mathrm{N}_{2} \mathrm{O}_{3}$ is $+3$
$\mathrm{N}_{2} \mathrm{O}_{3} 2 \mathrm{x}+3(-2)=0$
$x=+3$
