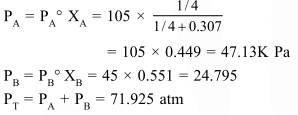Question:
On mixing, heptane and octane form an ideal solution. At $373 \mathrm{~K}$, the vapour pressures of the two liquid components (heptane and octane) are $105 \mathrm{kPa}$ and $45 \mathrm{kPa}$ respectively. Vapour pressure of the solution obtained by mixing $25.0$ of heptane and $35 \mathrm{~g}$ of octane will be (molar mass of heptane $=100 \mathrm{~g} \mathrm{~mol}^{-1}$ and of octane $=114 \mathrm{~g} \mathrm{~mol}^{-1}$ ) :-
Correct Option: , 2
Solution: