Question:
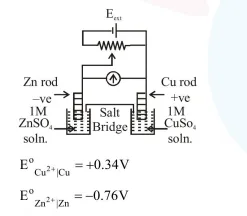
Identify the incorrect statement from the options below for the above cell:
Correct Option: 1,
Solution:
$\mathrm{E}_{\text {cell }}^{\mathrm{o}}=0.34-(-0.76)$
$=1.10 \mathrm{volt}$
If $E_{e x t}>1.10$ volt
$\mathrm{Cu} \rightarrow$ Anode
$\mathrm{Zn} \rightarrow$ Cathode
If $\mathrm{E}_{\mathrm{ext}}=1.10$ volt
$\mathrm{Zn} \rightarrow$ Anode
$\mathrm{Cu} \rightarrow$ Cathode
