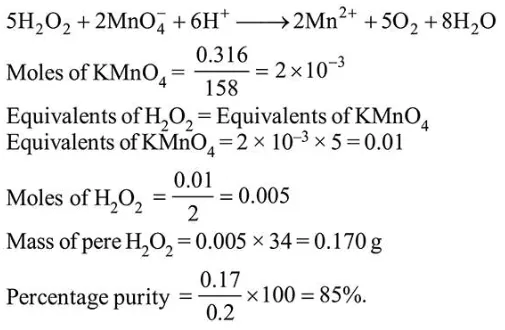
Question:
A $20.0 \mathrm{~mL}$ solution containing $0.2 \mathrm{~g}$ impure $\mathrm{H}_{2} \mathrm{O}_{2}$ reacts completely with $0.316 \mathrm{~g}$ of $\mathrm{KMnO}_{4}$ in acid solution. The purity of $\mathrm{H}_{2} \mathrm{O}_{2}$ (in \%) is________________ (mol. wt. of $\mathrm{H}_{2} \mathrm{O}_{2}=34$; mol. wt. of $\mathrm{KMnO}_{4}=1 \overline{58)}$
Solution:
(85)