Question:
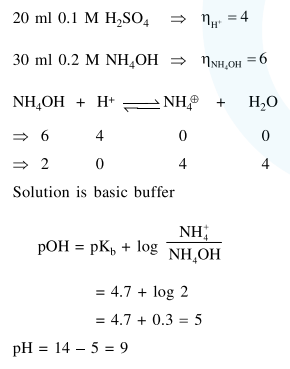
$20 \mathrm{~mL}$ of $0.1 \mathrm{MH}_{2} \mathrm{SO}_{4}$ solution is added to 30 $\mathrm{mL}$ of $0.2 \mathrm{M} \mathrm{NH}_{4} \mathrm{OH}$ solution. The $\mathrm{pH}$ of the resulatant mixture is : $\left[\mathrm{pk}_{\mathrm{b}}\right.$ of $\left.\mathrm{NH}_{4} \mathrm{OH}=4.7\right]$.
Correct Option: , 3
Solution: