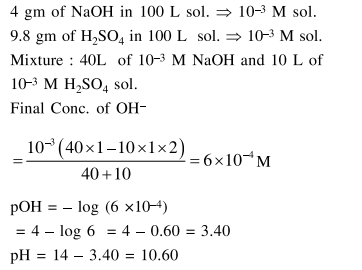Question:
Two solutions $\mathrm{A}$ and $\mathrm{B}$, each of $100 \mathrm{~L}$ was made by dissolving $4 \mathrm{~g}$ of $\mathrm{NaOH}$ and $9.8 \mathrm{~g}$ of $\mathrm{H}_{2} \mathrm{SO}_{4}$ in water, respectively. The $\mathrm{pH}$ of the resultant solution obtained from mixing $40 \mathrm{~L}$ of solution $\mathrm{A}$ and $10 \mathrm{~L}$ of solution B is
Solution: