Question:
The $K_{\mathrm{sp}}$ for the following dissociation is $1.6 \times 10^{-5}$
$\mathrm{PbCl}_{2(\mathrm{~s})} \rightleftharpoons \mathrm{Pb}_{(\mathrm{aq})}^{2+}+2 \mathrm{Cl}_{(\mathrm{aq})}^{-}$
Which of the following choices is correct for a mixture of $300 \mathrm{~mL} 0.134 \mathrm{M} \mathrm{Pb}\left(\mathrm{NO}_{3}\right)_{2}$ and $100 \mathrm{~mL} 0.4 \mathrm{M} \mathrm{NaCl}$ ?
Correct Option: , 2
Solution:
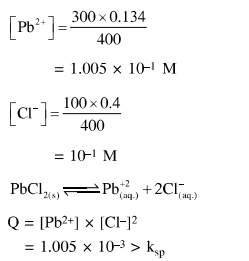
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
