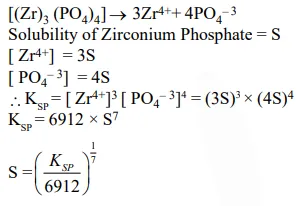Question:
Zirconium phosphate $\left[\mathrm{Zr}_{3}\left(\mathrm{PO}_{4}\right)_{4}\right]$ dissociates into three zirconium cations of charge $+4$ and four phosphate anions of charge $-3$. If molar solubility of zirconium phosphate is denoted by $\mathrm{S}$ and its solubility product by $\mathrm{K}_{\mathrm{sp}}$ then which of the following relationship between $\mathrm{S}$ and $\mathrm{K}_{\mathrm{sp}}$ is correct
Correct Option: , 3
Solution: