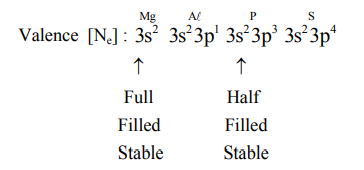
Question: The CORRECT order of first ionisation enthalpy is:
$\mathrm{Mg}<\mathrm{S}<\mathrm{Al}<\mathrm{P}$
$\mathrm{Mg}<\mathrm{Al}<\mathrm{S}<\mathrm{P}$
$\mathrm{Al}<\mathrm{Mg}<\mathrm{S}<\mathrm{P}$
$\mathrm{Mg}<\mathrm{Al}<\mathrm{P}<\mathrm{S}$
Correct Option: 3,
Solution:
$\mathrm{Mg} \mathrm{Al} \quad \mathrm{P} \quad \mathrm{S} \rightarrow \mathrm{IE}$. order $\Rightarrow \mathrm{Al}<\mathrm{Mg}<\mathrm{S}<\mathrm{P}$