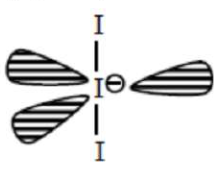
Question:
The correct shape and I-I-I bond angles respectively in $\mathrm{I}_{3}^{-}$ion are :
Correct Option: , 3
Solution:
$\mathrm{I}_{3}^{-} \mathrm{sp}^{3} \mathrm{~d}$ hybridisation (2BP + 3L.P.) Linear geometry