Question:
The d-electron configuration of $\left[\mathrm{Ru}(\mathrm{en})_{3}\right] \mathrm{Cl}_{2}$ and $\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right] \mathrm{Cl}_{2}$, respectively are :
Correct Option: 3,
Solution:
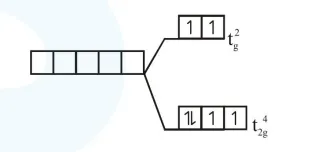
$\left[\mathrm{Ru}(\mathrm{en})_{3}\right] \mathrm{Cl}_{2}$
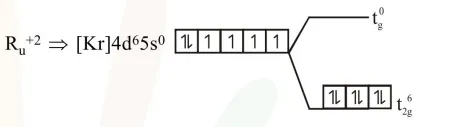
$\mathrm{R}_{\mathrm{u}} \Rightarrow 4 \mathrm{~d}$ series
en $\Rightarrow$ chelating ligand
$\mathrm{CN}=6$, octahedral splitting hence laye splitting of d-subshell
$\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right] \mathrm{Cl}_{2} \Rightarrow \mathrm{H}_{2} \mathrm{O} \Rightarrow$ Weak filled ligand
$\mathrm{Fe}^{+2} \Rightarrow[\mathrm{Ar}] 3 \mathrm{~d}^{6} 4 \mathrm{~s}^{0}$
less plitting
$\mathrm{CN}=6$ octahedral splitting