Question:
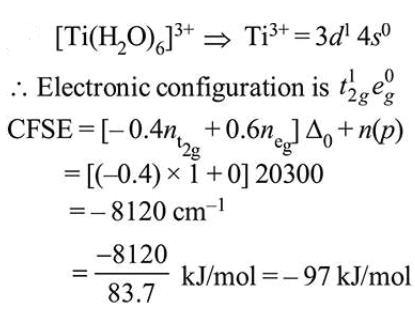
The electronic spectrum of $\left[\mathrm{Ti}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{3+}$ shows a single broad peak with a maximum at $20,300 \mathrm{~cm}^{-1}$. The crystal field stabilization energy (CFSE) of the complex ion, in $\mathrm{kJ} \mathrm{mol}^{-1}$, is : $\left(1 \mathrm{~kJ} \mathrm{~mol}^{-1}=83.7 \mathrm{~cm}^{-1}\right)$
Correct Option:
Solution:
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
