The equilibrium constant for the following reaction is $1.6 \times 10^{5}$ at $1024 \mathrm{~K}$.
$\mathrm{H}_{2}(\mathrm{~g})+\mathrm{Br}_{2}(\mathrm{~g}) \longleftrightarrow 2 \mathrm{HBr}(\mathrm{g})$
Find the equilibrium pressure of all gases if $10.0$ bar of $\mathrm{HBr}$ is introduced into a sealed container at $1024 \mathrm{~K}$.
Given,
$K_{\mathrm{p}}$ for the reaction i.e., $\mathrm{H}_{2(g)}+\mathrm{Br}_{2(g)} \longleftrightarrow 2 \mathrm{HBr}_{(g)}$ is $1.6 \times 10^{5}$.
Therefore, for the reaction $2 \mathrm{HBr}_{(\mathrm{g})} \longleftrightarrow \mathrm{H}_{2(\mathrm{~g})}+\mathrm{Br}_{2(\mathrm{~g})}$, the equilibrium constant will be,
$K_{\mathrm{p}}^{\prime}=\frac{1}{K_{\mathrm{P}}}$
$=\frac{1}{1.6 \times 10^{5}}$
$=6.25 \times 10^{-6}$
Now, let $p$ be the pressure of both $\mathrm{H}_{2}$ and $\mathrm{Br}_{2}$ at equilibrium.
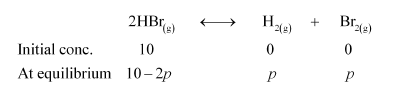
Now, we can write,
$\frac{p_{\mathrm{HB}_{1}} \times p_{2}}{p_{\mathrm{HBr}}^{2}}=K_{\mathrm{P}}^{\prime}$
$\frac{p \times p}{(10-2 p)^{2}}=6.25 \times 10^{-6}$
$\frac{p}{10-2 p}=2.5 \times 10^{-3}$
$p=2.5 \times 10^{-2}-\left(5.0 \times 10^{-3}\right) p$
$p+\left(5.0 \times 10^{-3}\right) p=2.5 \times 10^{-2}$
$\left(1005 \times 10^{-3}\right) p=2.5 \times 10^{-2}$
$p=2.49 \times 10^{-2} \mathrm{bar}=2.5 \times 10^{-2} \mathrm{bar}($ approximately $)$
Therefore, at equilibrium,
$\left[\mathrm{H}_{2}\right]=\left[\mathrm{Br}_{2}\right]=2.49 \times 10^{-2}$ bar
$[\mathrm{HBr}]=10-2 \times\left(2.49 \times 10^{-2}\right)$ bar
$=9.95 \mathrm{bar}=10 \mathrm{bar}($ approximately $)$
