Question:
The half life period of a first order chemical reaction is $6.93$ minutes. The time required for the completion of $99 \%$ of the chemical reaction will be $(\log 2=0.301):-$
Correct Option: 1
Solution:
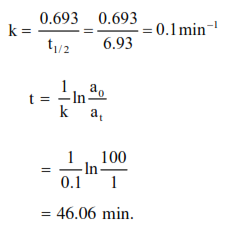
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
