Question:
The hybridization and magnetic nature of $\left[\mathrm{Mn}(\mathrm{CN})_{6}\right]^{4-}$ and $\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}$, respectively are:
Correct Option: 1
Solution:
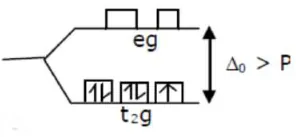
$1 .\left(\mathrm{Mn}(\mathrm{CN})_{6}\right)^{4-}$
$\mathrm{Mn}^{++}=3 \mathrm{~d}^{5}$
$\mu=\sqrt{3}$
hybridization $=\mathrm{d}^{2} \mathrm{sp}^{3}$
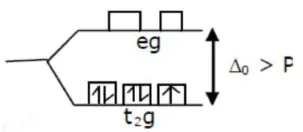
2. $\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}$
$\mathrm{Fe}^{3+}=3 \mathrm{~d}^{5}$
$\mu=\sqrt{3}$