Question:
The incorrect statement is :
Correct Option: 3,
Solution:
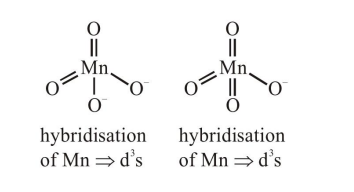
Option 1) Manganate $\Rightarrow \mathrm{MnO}_{4}^{2-}$,
Permanganate $\Rightarrow \mathrm{MnO}_{4}^{-}$
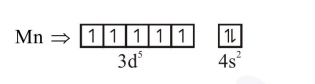
After excitation
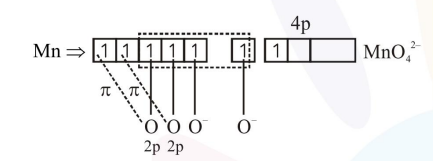
$2 \times 2 \mathrm{p}_{\pi}-3 \mathrm{~d}_{\pi \sigma}$
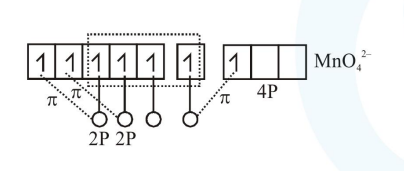
$2 \times 2 \mathrm{P}_{\pi}-3 \mathrm{~d}_{\pi}$
$1 \times 2 \mathrm{P}_{\pi}-4 \mathrm{P}_{\pi}$
(2) $\mathrm{MnO}_{4}^{2-} \Rightarrow$ green
$\mathrm{MnO}_{4}^{-} \Rightarrow$ purple/violet
(3) Manganate contains 1 unpaired electron hence it is paramagnetic
where as permanganetic contains no unpaired electrons hence it is diamagnetic.
(4) Both have $\mathrm{d}^{3} \mathrm{~s}$ hybridisation hence both have tetrahedral geometry.
