Question:
The increasing order of pKa of the following amino acids in aqueous solution is:
Gly Asp Lys Arg
Correct Option:
Solution:
Structure of the given $\alpha$-amino acids are:
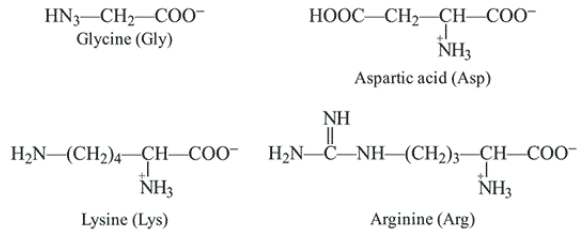
Here, aspartic acid is an acidic and glycine is a neutral amino acid while lysine and arginine are basic amino acids. Also, arginine is more basic due to the stronger basic functional groups.
$\therefore$ The order of $p K a$ value is directly proportional to the basic strength of amino acids, i.e. Arg $>$ Lys $>$Gly > Asp.
