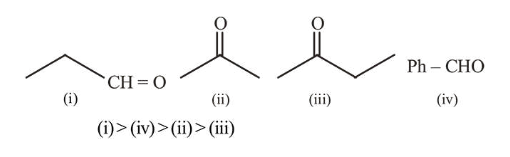
The increasing order of the reactivity of the following compounds in nucleophilic addition reaction is :
Question:
The increasing order of the reactivity of the following compounds in nucleophilic addition reaction is :
Propanal, Benzaldehyde, Propanone, Butanone
Correct Option: , 2
Solution:
Rate of Nucleophillic addition reaction is directly proportional to the $-I$ and $-M$ effect of the substituents present in the substrate. Ketones are less susceptible to the nucleophillic addition, due to the presence of alkyl (R) group which has +I effect. Thus reactivity order
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
