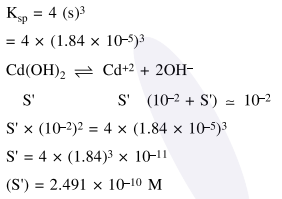Question: The molar solubility of $\mathrm{Cd}(\mathrm{OH})_{2}$ is $1.84 \times 10^{-5} \mathrm{M}$ in water. The expected solubility of $\mathrm{Cd}(\mathrm{OH})_{2}$ in a buffer solution of $\mathrm{pH}=12$ is :
$6.23 \times 10^{-11} \mathrm{M}$
$1.84 \times 10^{-9} \mathrm{M}$
$\frac{2.49}{1.84} \times 10^{-9} \mathrm{M}$
$2.49 \times 10^{-10} \mathrm{M}$
Correct Option: , 4
Solution: