Question:
The number of isomers possible for $\left[\mathrm{Pt}(\mathrm{en})\left(\mathrm{NO}_{2}\right)_{2}\right]$ is :
Correct Option: 1
Solution:
$\left[\mathrm{Pt}(\right.$ en $\left.)\left(\mathrm{NO}_{2}\right)_{2}\right] \Rightarrow$ does not show G.I. as well as optical isomerism.
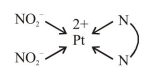
This complex will have three linkage isomers as follows :-
[Pt (en) $\left.\left(\mathrm{NO}_{2}\right) 2\right] \mathrm{I}$
[Pt (en) $\left.\left(\mathrm{NO}_{2}\right)(\mathrm{ONO})\right] \mathrm{II}$
$\left[\mathrm{Pt}(\mathrm{en})(\mathrm{ONO})_{2}\right] \mathrm{III}$
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
