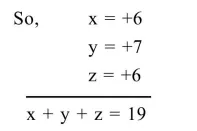Question:
The oxidation states of transition metal atoms in $\mathrm{K}_{2} \mathrm{Cr}_{2} \mathrm{O}_{7}$, $\mathrm{KMnO}_{4}$ and $\mathrm{K}_{2}\mathrm{FeO}_{4}$, respectively, are $x, y$ and $z$. The sum of $x, y$ and $z$ is_________.
Solution:
$\mathrm{K}_{2} \mathrm{Cr}_{2} \mathrm{O}_{7}$
$2(+1)+2 x+7(-2)=0$
$x=+6$
In $\mathrm{K}_{2} \mathrm{Cr}_{2} \mathrm{O}_{7}$, Transition metal (Cr) present in $+6$ oxidation state.
$\mathrm{KMnO}_{4}$
$(+1)+y+4(-2)=0$
$x=+7$
In $\mathrm{KMnO}_{4}$, transition metal $(\mathrm{Mn})$ present in $+7$ oxidation state
$\mathrm{K}_{2} \mathrm{FeO}_{4}$
$2(+1)+z+4(-2)=0$
$x=+6$
In $\mathrm{K}_{2} \mathrm{FeO}_{4}$, transition metal (Fe) present in $+6$ oxidation state