Question:
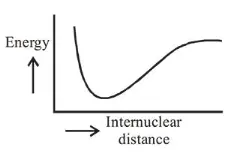
The potential energy curve for the $\mathrm{H}_{2}$ molecule as a function of internuclear distance is :
Correct Option: , 2
Solution:
When two H-atoms come closer then initially due to attraction P.E. is -ve, which decreases more as atoms come closer and after reacting to a minimum value as repulsion starts dominating. So, P.E. increases then.