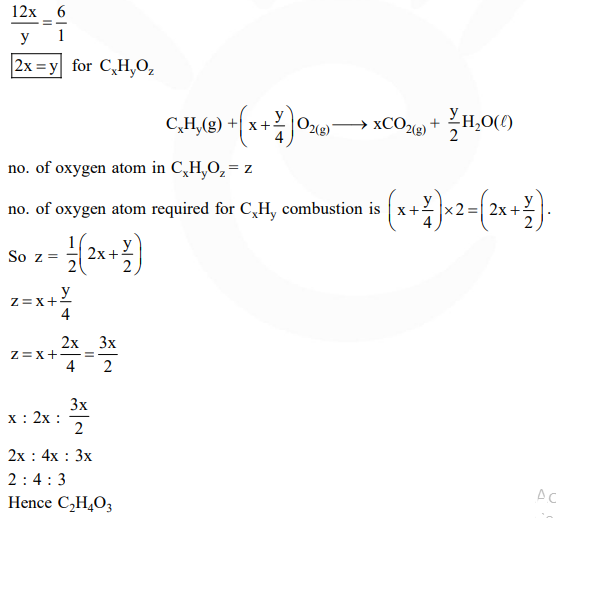
The ratio of mass percent of $\mathrm{C}$ and $\mathrm{H}$ of an organic compound $\left(\mathrm{C}_{\mathrm{X}} \mathrm{H}_{\mathrm{Y}} \mathrm{O}_{\mathrm{Z}}\right)$ is $6: 1$. If one molecule of the above compound $\left(\mathrm{C}_{\mathrm{X}} \mathrm{H}_{\mathrm{Y}} \mathrm{O}_{\mathrm{Z}}\right)$ contains half as much oxygen as required to burn one molecule of compound $\mathrm{C}_{\mathrm{X}} \mathrm{H}_{\mathrm{Y}}$ completely to $\mathrm{CO}_{2}$ and $\mathrm{H}_{2} \mathrm{O}$. The empirical formula of compound $\mathrm{C}_{\mathrm{X}} \mathrm{H}_{\mathrm{Y}} \mathrm{O}_{\mathrm{Z}}$ is
Correct Option:
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
