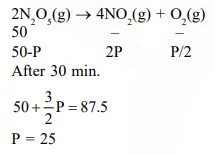Question:
The reaction
$2 \mathrm{~N}_{2} \mathrm{O}_{5}(\mathrm{~g}) \rightarrow 4 \mathrm{NO}_{2}(\mathrm{~g})+\mathrm{O}_{2}(\mathrm{~g})$
follows first order kinetics. The pressure of a vessel containing only $\mathrm{N}_{2} \mathrm{O}_{5}$ was found to increase from $50 \mathrm{~mm} \mathrm{Hg}$ to $87.5 \mathrm{~mm} \mathrm{Hg}$ in $30 \mathrm{~min}$. The pressure exerted by the gases after $60 \mathrm{~min}$.
Correct Option: 1
Solution: