Question:
The solubility of $\mathrm{CdSO}_{4}$ in water is $8.0 \times 10^{-4} \mathrm{~mol} \mathrm{~L}^{-1}$. Its solubility in $0.01 \mathrm{M}$ $\mathrm{H}_{2} \mathrm{SO}_{4}$ solution is_________ $\times 10^{-6} \mathrm{~mol} \mathrm{~L}^{-1}$.
(Round off to the Nearest integer) (Assume that solubility is much less than $0.01 \mathrm{M}$ )
Solution:
In pure water,
$\mathrm{K}_{\mathrm{sp}}=\mathrm{S}^{2}=\left(8 \times 10^{-4}\right)^{2}$
$=64 \times 10^{-8}$
In $0.01 \mathrm{M} \mathrm{H}_{2} \mathrm{SO}_{4}$
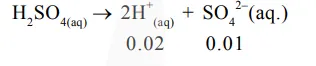
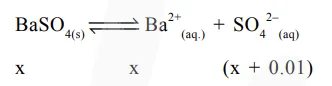
$\mathrm{K}_{\mathrm{sp}}=\mathrm{x}(\mathrm{x}+0.01)$
$=64 \times 10^{-8}$
$\mathrm{x}+0.01 \cong 0.01 \mathrm{M}$
So, $x(0.01)=64 \times 10^{-8}$
$x=64 \times 10^{-6} \mathrm{M}$
