Question:
The species: $\mathrm{H}_{2} \mathrm{O}, \mathrm{HCO}_{7}^{-}, \mathrm{HSO}_{4}^{-}$, and $\mathrm{NH}_{3}$ can act both as Brönsted acids and bases. For each case give the corresponding conjugate acid and base.
Solution:
The table below lists the conjugate acids and conjugate bases for the given species.
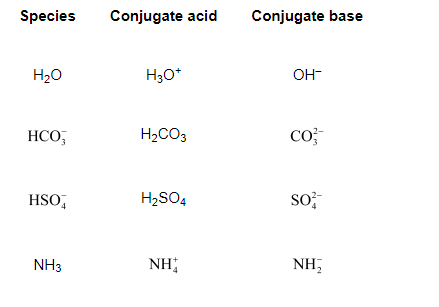
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
