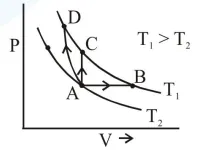
Three different processes that can occur in an ideal monoatomic gas are shown in the $\mathrm{P}$ vs $\mathrm{V}$ diagram. The paths are labelled as $\mathrm{A} \rightarrow \mathrm{B}, \mathrm{A} \rightarrow \mathrm{C}$ and $\mathrm{A} \rightarrow \mathrm{D}$. The change in internal energies during these process are taken as $\mathrm{E}_{\mathrm{AB}}, \mathrm{E}_{\mathrm{AC}}$ and $\mathrm{E}_{\mathrm{AD}}$ and the workdone as $\mathrm{W}_{\mathrm{AB}}$, $\mathrm{W}_{\mathrm{AC}}$ and $\mathrm{W}_{\mathrm{AD}}$. The correct relation between these parameters are :
Correct Option: 1
$\Delta \mathrm{U}=\mathrm{nC}_{\mathrm{y}} \Delta \mathrm{T}=$ same
$\mathrm{AB} \rightarrow$ volume is increasing $\Rightarrow \mathrm{W}>0$
$\mathrm{AD} \rightarrow$ volume is decreasing $\Rightarrow \mathrm{W}<0$
$\mathrm{AC} \rightarrow$ volume is constant $\Rightarrow \mathrm{W}=0$
