Use R=8.3 J/mol-K wherever required. 50 cc of oxygen is collected in an inverted gas jar over water.
Question:
Use $\mathrm{R}=8.3 \mathrm{~J} / \mathrm{mol}-\mathrm{K}$ wherever required.
$50 \mathrm{cc}$ of oxygen is collected in an inverted gas jar over water. The atmospheric pressure is $99.4 \mathrm{kPa}$ and the room temperature is $27^{\circ} \mathrm{C}$. The water level in the jar is same as the level outside. The saturation vapour pressure at $27^{\circ} \mathrm{C}$ is $3.4 \mathrm{kPa}$. Calculate the number of moles of oxygen collected in the jar.
Solution:
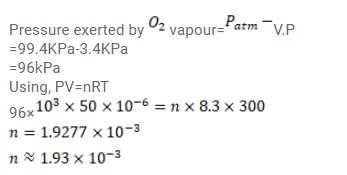
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
