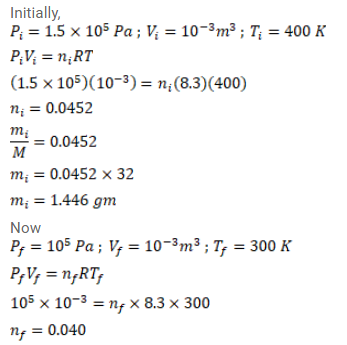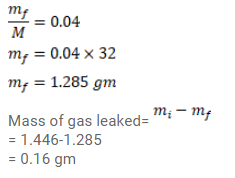Question:
Use $\mathrm{R}=8.3 \mathrm{~J} / \mathrm{mol}-\mathrm{K}$ wherever required.
Oxygen is filled in a closed jar of volume $1.0 \times 10^{-3} \mathrm{~m}^{3}$ at a pressure of $1.5 \times 10^{5} \mathrm{~Pa}$ and temperature $400 \mathrm{~K}$. The jar has a small leak in it. The atmospheric pressure is $1.0 \times 10^{5} \mathrm{~Pa}$ and the atmospheric temperature is $300 \mathrm{~K}$. Find the mass of the gas that leaks out by the time the pressure and the temperature inside the jar equalize with the surrounding.
Solution: