Question:
What happens when zinc granules are treated with dilute solution of H2SO4, HCl, HNO3, NaCl and NaOH ? Also write the chemical equations if reaction
oçcurs.
Solution:
With dilute H2SO4 : Hydrogen gas evolves
Zn (s) + H2SO4 (dil.) ————> ZnSO4 (aq) + H2(g)
with dilute HCl : Hydrogen gas evolves
Zn (s) + 2HCl (dil.) ———–> ZnCl2(aq) + H2(g)
with dilute HNO3 : Nitrous oxide gas evolves. It is colourless.
4Zn (s) + 10HNO3 (aq) ———-> 4Zn(NO3)2 (aq) + 5H2O (l) + N2O(g)
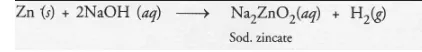
with NaCl : There is no chemical reaction, with NaOH : Hydrogen gas evolves :