Question.
What is a neutralisation reaction ? Give two examples.
What is a neutralisation reaction ? Give two examples.
solution:
A reaction in which an acid and base react with each other to give a salt and water is termed as neutralisation reaction. In this reaction, energy is evolved in the form of heat.
For example,
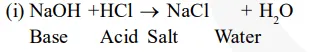
(ii) During indigestion (caused due to the production of excess of hydrochloric acid in the stomach),we administer an antacid (generally milk of magnesia, $\mathrm{Mg}(\mathrm{OH})_{2}$ which is basic in nature). The antacid neutralises the excess of acids and thus gives relief from indigestion.
$\mathrm{Mg}(\mathrm{OH})_{2}+2 \mathrm{HCl} \rightarrow \mathrm{MgCl}_{2}+2 \mathrm{H}_{2} \mathrm{O}$
A reaction in which an acid and base react with each other to give a salt and water is termed as neutralisation reaction. In this reaction, energy is evolved in the form of heat.
For example,
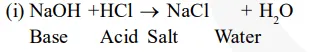
(ii) During indigestion (caused due to the production of excess of hydrochloric acid in the stomach),we administer an antacid (generally milk of magnesia, $\mathrm{Mg}(\mathrm{OH})_{2}$ which is basic in nature). The antacid neutralises the excess of acids and thus gives relief from indigestion.
$\mathrm{Mg}(\mathrm{OH})_{2}+2 \mathrm{HCl} \rightarrow \mathrm{MgCl}_{2}+2 \mathrm{H}_{2} \mathrm{O}$
