What is the concentration of sugar $\left(\mathrm{C}_{12} \mathrm{H}_{22} \mathrm{O}_{11}\right)$ in $\mathrm{mol} \mathrm{L}^{-1}$ if its $20 \mathrm{~g}$ are dissolved in enough
Question.
What is the concentration of sugar $\left(\mathrm{C}_{12} \mathrm{H}_{22} \mathrm{O}_{11}\right)$ in $\mathrm{mol} \mathrm{L}^{-1}$ if its $20 \mathrm{~g}$ are dissolved in enough
water to make a final volume up to 2 L?
What is the concentration of sugar $\left(\mathrm{C}_{12} \mathrm{H}_{22} \mathrm{O}_{11}\right)$ in $\mathrm{mol} \mathrm{L}^{-1}$ if its $20 \mathrm{~g}$ are dissolved in enough
water to make a final volume up to 2 L?
Solution:
Molarity (M) of a solution is given by
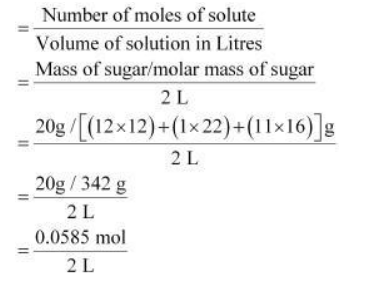
$=0.02925 \mathrm{~mol} \mathrm{~L}^{-1}$
$\therefore$ Molar concentration of sugar $=0.02925 \mathrm{~mol} \mathrm{~L}^{-1}$
Molarity (M) of a solution is given by

$=0.02925 \mathrm{~mol} \mathrm{~L}^{-1}$
$\therefore$ Molar concentration of sugar $=0.02925 \mathrm{~mol} \mathrm{~L}^{-1}$
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
