What is the equilibrium concentration of each of the substances in the equilibrium when the initial concentration of ICl was 0.78 M?
What is the equilibrium concentration of each of the substances in the equilibrium when the initial concentration of ICl was 0.78 M?
$2 \mathrm{ICl}(\mathrm{g}) \rightleftharpoons \mathrm{I}_{2}(\mathrm{~g})+\mathrm{Cl}_{2}(\mathrm{~g}) ; \mathrm{K}_{\mathrm{C}}=0.14$
The given reaction is:
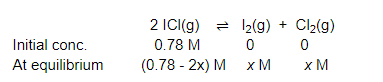
Now, we can write, $\frac{\left[\mathrm{I}_{2}\right]\left[\mathrm{Cl}_{2}\right]}{[\mathrm{ICl}]^{2}}=K_{\mathrm{C}}$
$\Rightarrow \frac{x \times x}{(0.78-2 x)^{2}}=0.14$
$\Rightarrow \frac{x^{2}}{(0.78-2 x)^{2}}=0.14$
$\Rightarrow \frac{x}{0.78-2 x}=0.374$
$\Rightarrow x=0.292-0.748 x$
$\Rightarrow 1.748 x=0.292$
$\Rightarrow x=0.167$
Hence, at equilibrium,
$[\mathrm{ICl}]=\left[\mathrm{I}_{2}\right]=0.167 \mathrm{M}$
$[\mathrm{ICl}]=(0.78-2 \times 0.167) \mathrm{M}$
$=0.446 \mathrm{M}$
