Question.
What is the maximum number of emission lines when the excited electron of an H atom in n = 6 drops to the ground state?
What is the maximum number of emission lines when the excited electron of an H atom in n = 6 drops to the ground state?
Solution:
When the excited electron of an H atom in n = 6 drops to the ground state, the following transitions are possible:
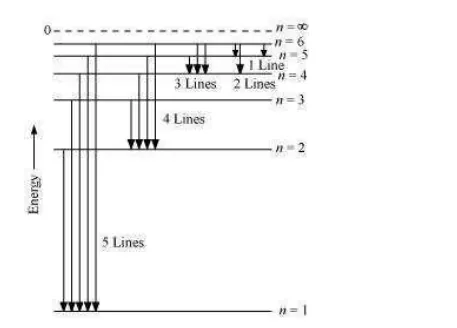
Hence, a total number of (5 + 4 + 3 + 2 + 1) 15 lines will be obtained in the emission spectrum.
The number of spectral lines produced when an electron in the $n^{\text {th }}$ level drops down to
the ground state is given by $\frac{n(n-1)}{2}$
Given, n = 6
Number of spectral lines $=\frac{6(6-1)}{2}=15$
When the excited electron of an H atom in n = 6 drops to the ground state, the following transitions are possible:

Hence, a total number of (5 + 4 + 3 + 2 + 1) 15 lines will be obtained in the emission spectrum.
The number of spectral lines produced when an electron in the $n^{\text {th }}$ level drops down to
the ground state is given by $\frac{n(n-1)}{2}$
Given, n = 6
Number of spectral lines $=\frac{6(6-1)}{2}=15$
