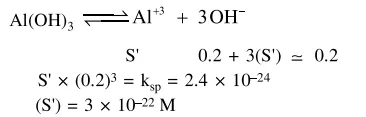Question: What is the molar solubility of $\mathrm{Al}(\mathrm{OH})_{3}$ in $0.2 \mathrm{M} \mathrm{NaOH}$ solution ? Given that, solubility product of $\mathrm{Al}(\mathrm{OH})_{3}=2.4 \times 10^{-24}$ :
$12 \times 10^{-23}$
$12 \times 10^{-21}$
$3 \times 10^{-19}$
$3 \times 10^{-22}$
Correct Option: , 4
Solution: