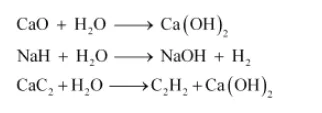Question:
What properties of water make it useful as a solvent? What types of compound can it
(i) dissolve, and
(ii) hydrolyse?
Solution:
A high value of dielectric constants (78.39 C2/Nm2) and dipole moment make water a universal solvent.
Water is able to dissolve most ionic and covalent compounds. Ionic compounds dissolve in water because of the ion-dipole interaction, whereas covalent compounds form hydrogen bonding and dissolve in water.
Water can hydrolyze metallic and non-metallic oxides, hydrides, carbides, phosphides, nitrides and various other salts. During hydrolysis, H+ and OH– ions of water interact with the reacting molecule.
Some reactions are: