Question:
Which one of the following depicts the correct representation of atomic radius (r) of an atom ?
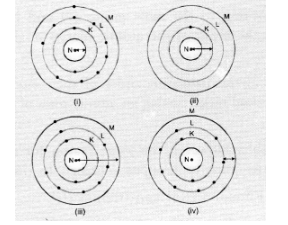
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (iii) and (iv)
(d) (i) and (iv).
Solution:
(b). The atom (ii) has only one shell (K-shell). No electrons are present in the other shells. Therefore, the arrow represents correct atomic radius.
The arrow in atom (iii) also represents the correct atomic radius of the element.
